Target
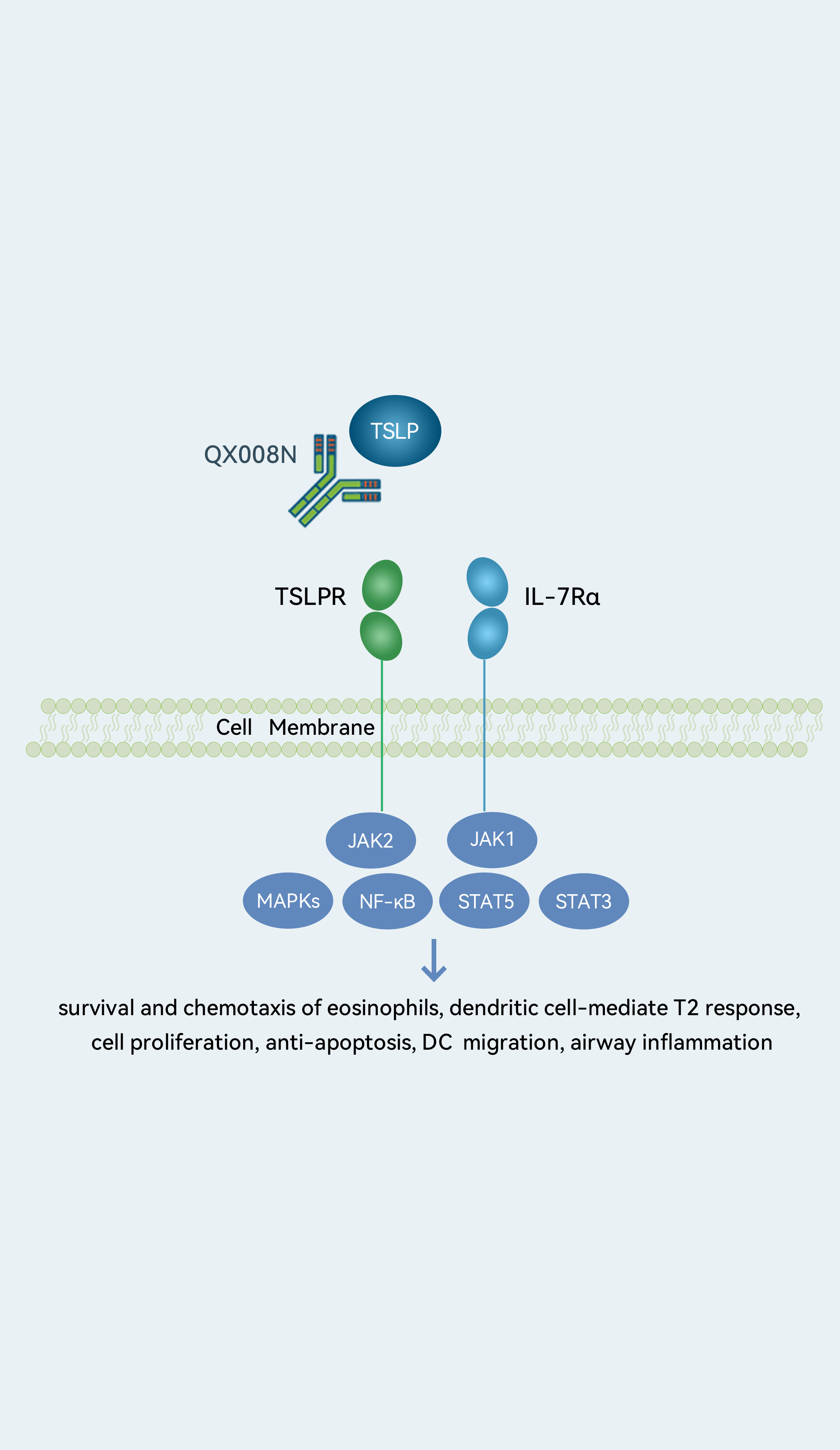
Target- TSLP
 Generic Name
Generic Name- Recombinant humanized anti TSLP monoclonal antibody injection
 Indication
Indication- asthma moderate-to-severe COPD severe asthma
We are developing QX008N, a humanized IgG1 mAb targeting TSLP, for asthma and moderate-to-severe COPD. TSLP-targeting therapy is the only class of biologic drugs globally approved for asthma that can slow disease progression for asthma patients with low-level or no expression of type 2 biomarkers. QX008N demonstrated a potency superior to an internally prepared tezepelumab analog and exhibited a good safety profile based on preliminary results from our Phase Ia clinical trial. We are conducting a Phase Ia clinical trial in healthy subjects, which we expect to complete in the second quarter of 2023, and plan to initiate a Phase Ib clinical trial in the meantime.
According to Frost & Sullivan, the prevalence of asthma and COPD in China was 65.9 million and 105.7 million in 2021, respectively, and is estimated to reach 78.1 million and 110.8 million in 2030, respectively. The asthma drug market in China was US$4.0 billion in 2021, and is estimated to reach US$10.7 billion in 2030, at a CAGR of 11.6%. The COPD drug market in China was US$3.3 billion in 2021, and is estimated to reach US$7.4 billion in 2030, at a CAGR of 9.6%. As of March 2023, omalizumab was the only biologic drug for asthma approved in China and no biologic drug had been approved for the treatment of COPD in China.